Introduction
Human behavior arises from a profoundly complex interplay between genes and environment. Within that interplay, chromosomal anomalies, structural or numerical deviations from the normal set of 46 chromosomes, can exert powerful influences on brain development, cognition, temperament, and behavior. While not all behavioral differences are rooted in genetics, chromosomal aberrations help explain many neurodevelopmental and psychiatric phenotypes observed across the lifespan. This guide critically evaluates the genetics–behavior nexus through the lens of chromosomal anomalies, synthesizing recent research, clinical insights, and actionable understanding.
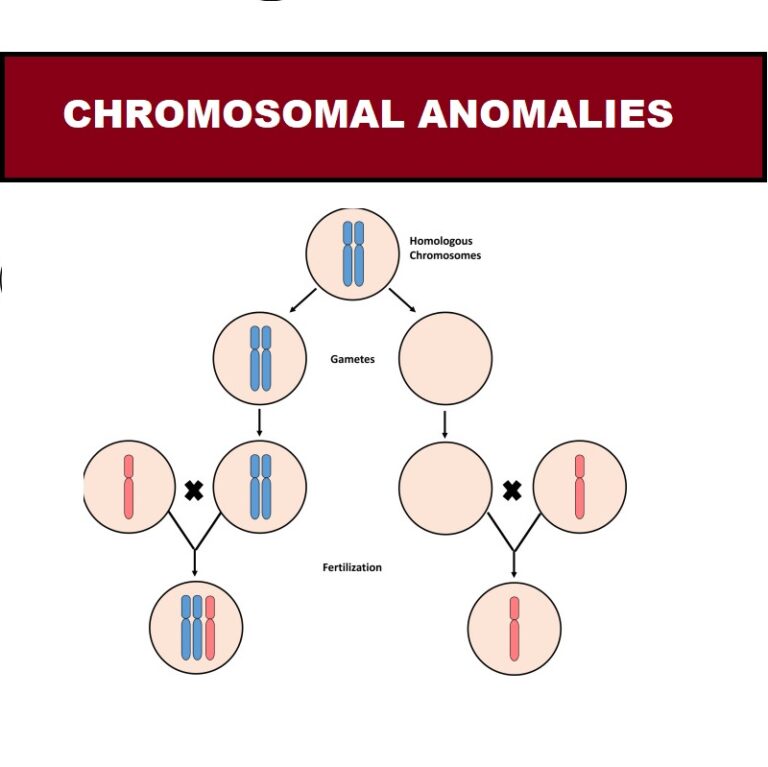
1. What Are Chromosomal Anomalies?
Chromosomal anomalies are deviations in chromosome number (aneuploidies) or structure (deletions, duplications, translocations) that can disrupt gene function.
Types of Chromosomal Anomalies
| Category | Example | Potential Behavioral Impact |
|---|---|---|
| Aneuploidy | 47,XXY (Klinefelter) | Social communication challenges; mood dysregulation |
| Microdeletion/Microduplication | 16p11.2 deletion | Increased risk for autism spectrum behaviors; intellectual disability |
| Balanced Rearrangement | Translocation carriers | Usually asymptomatic, but risk of affected offspring increases |
| Unbalanced Rearrangement | Complex structural aberrations | Global neurodevelopmental delay; epilepsy |
Chromosomal microarrays and next-generation sequencing have substantially improved detection of submicroscopic structural variants called copy number variations (CNVs), which can disrupt networks involved in behavior and cognition even when routine karyotyping appears normal.
2. Chromosomal Anomalies and Neurodevelopmental Behavior
Many behavioral and cognitive syndromes trace directly to chromosomal changes that disrupt key brain development pathways.
2.1 Autism Spectrum Disorder (ASD)
ASD is characterized by social communication differences and repetitive patterns of behavior. Genetics play a major role: recent large studies have identified hundreds of chromosomal and gene variants associated with ASD:
- Chromosomal microarrays detect CNVs in individuals with ASD and intellectual disability, affirming that structural changes contribute to risk.
- Novel rare genetic variants have been linked to ASD, some traceable through fetal ultrasonography anomalies and confirmed by whole-exome sequencing, underscoring prenatal genetic influences on behavior.
- Genome sequencing studies in diverse populations have identified candidate autism risk genes that may help clarify behavioral manifestations of chromosomal disruption.
These findings emphasize that ASD is heterogeneous; not a single condition but rather a cluster of neurodevelopmental pathways affected by different genetic and chromosomal factors.
Practical Insight: Clinicians assessing children with ASD symptoms should consider chromosomal microarray testing early, as identifying CNVs can guide tailored support plans, anticipate comorbidities, and inform family planning.
2.2 16p11.2 Deletion Syndrome
One well-studied microdeletion region — 16p11.2 — illustrates how structural chromosome variants can influence behavior. Individuals with this deletion often show:
- Global developmental delay
- Language and motor coordination challenges
- Psychiatric conditions including ASD features and mood instability
This microdeletion occurs spontaneously in many cases but may be inherited in families with a history of neurodevelopmental disorders. Its behavioral effects vary widely even among people with the same deletion, reflecting the complex interplay of genetic background and environmental context.
Practical Insight: Early developmental and behavioral intervention — such as speech therapy and structured social skills training — often yields better outcomes than waiting for traditional diagnoses alone.
3. How Chromosomal Anomalies Alter Brain Development and Behavior
Chromosomal anomalies impact behavior through several mechanisms:
3.1 Gene Dosage and Neurocircuits
Deletion or duplication changes the dosage of multiple genes, altering the balance of proteins involved in synaptic development, neural migration, and neurotransmitter systems. Many CNVs associated with ASD and ADHD disrupt genes highly expressed in the growing brain.
3.2 Disruption of Regulatory Networks
Chromosomal rearrangements can reposition regulatory elements, thereby affecting the expression of genes far from the breakpoint location — an effect known as position effect.
3.3 Mosaicism
In some individuals, not all cells carry the chromosomal change (mosaicism), leading to variable behavioral expression within a single disorder.
4. Behavioral Phenotypes Linked to Chromosomal Syndromes
A useful concept in clinical genetics is the behavioral phenotype — the characteristic pattern of behavior associated with a specific genetic condition. For example:
- Down syndrome (Trisomy 21) often shows strengths in social engagement but relative weaknesses in expressive language.
- Prader-Willi syndrome (15q11–q13 deletion) involves hyperphagia and temper tantrums alongside cognitive challenges.
- Williams syndrome (7q11.23 deletion) is associated with unusually friendly behavior and strong language skills but poor visuospatial cognition.
Knowing behavioral phenotypes helps clinicians anticipate needs and tailor interventions.
5. Practical Guidance for Genetics-Behavior Evaluation
5.1 Clinical Assessment Workflow
- Detailed history and developmental screening.
- Referral for genetic counseling when neurodevelopmental differences, dysmorphic features, or family history are present.
- Chromosomal microarray analysis (CMA) is first-line for detecting CNVs associated with behavior variability.
- Targeted gene panels or whole-exome sequencing when CMA is uninformative but suspicion remains high.
5.2 Interpreting Results
- A pathogenic CNV supports a diagnosis but does not fully determine behavior; environment and other genes matter.
- Variants of uncertain significance (VUS) require cautious interpretation and may be reclassified with growing evidence.
5.3 Family Support
Genetic results can be emotionally overwhelming. Structured support that combines genetic counseling, behavioral therapy, and education planning yields the best long-term outcomes.
6. Future Research Frontiers
Cutting-edge genetic research is improving our understanding of chromosomal influences on behavior:
- Large population sequencing efforts continue to reveal novel variants that shape behavioral phenotypes.
- Translational models (e.g., animal models and neuronal cultures) are being used to dissect molecular pathways from genotype to behavior.
- Integration of advanced bioinformatics and deep learning is helping prioritize which chromosomal changes are likely to be causally linked to behavioral outcomes.
Conclusion
Behavior emerges from nuanced genetic architecture that includes chromosomal integrity as a key foundational layer. Chromosomal anomalies — whether large numerical changes or subtle microdeletions — can reshape neural development and behavior in measurable ways. By understanding the genetic mechanisms at play, clinicians, families, and educators can tailor early interventions, anticipate comorbidities, and support individuals in realizing their potential.
Harnessing the latest genomic technologies — from chromosomal microarrays to whole-exome sequencing — is now standard practice in evaluating behavioral differences, particularly when development deviates from expected norms. With continued research and expanding data across diverse populations, the connection between chromosomes and behavior will become ever clearer and more actionable.
References & Citations
- Regev, O., Shil, A., Bronshtein, T., et al. Association between rare, genetic variants linked to autism and fetal anomalies in children with autism spectrum disorder. J Neurodevelopmental Disorders (2024).
- Kılıçaslan, F., Öz, Ö., & Mutlu, M. B. Investigation of chromosomal anomalies in autism spectrum disorder by array CGH. Int J Dev Neurosci (2025).
- Chaves, T. F. et al. Neurodevelopmental disorders and congenital anomalies: chromosomal microarray insights. Sci Rep (2024).
- Salim, S., Khaleel, A., Alkrdoshi, M., et al. Cytogenetic study of autism: systematic review. Razi Med J (2025).
- La Monica, I., Di Iorio, M. R., Sica, A., et al. Autism spectrum disorder: genetic mechanisms and inheritance patterns. Genes (2025).
- Behavioral Phenotypes of Genetic Syndromes: A reference guide. J Am Acad Child Adolesc Psychiatry (2001).
- Data on the 16p11.2 deletion syndrome and associated behavioral features.